
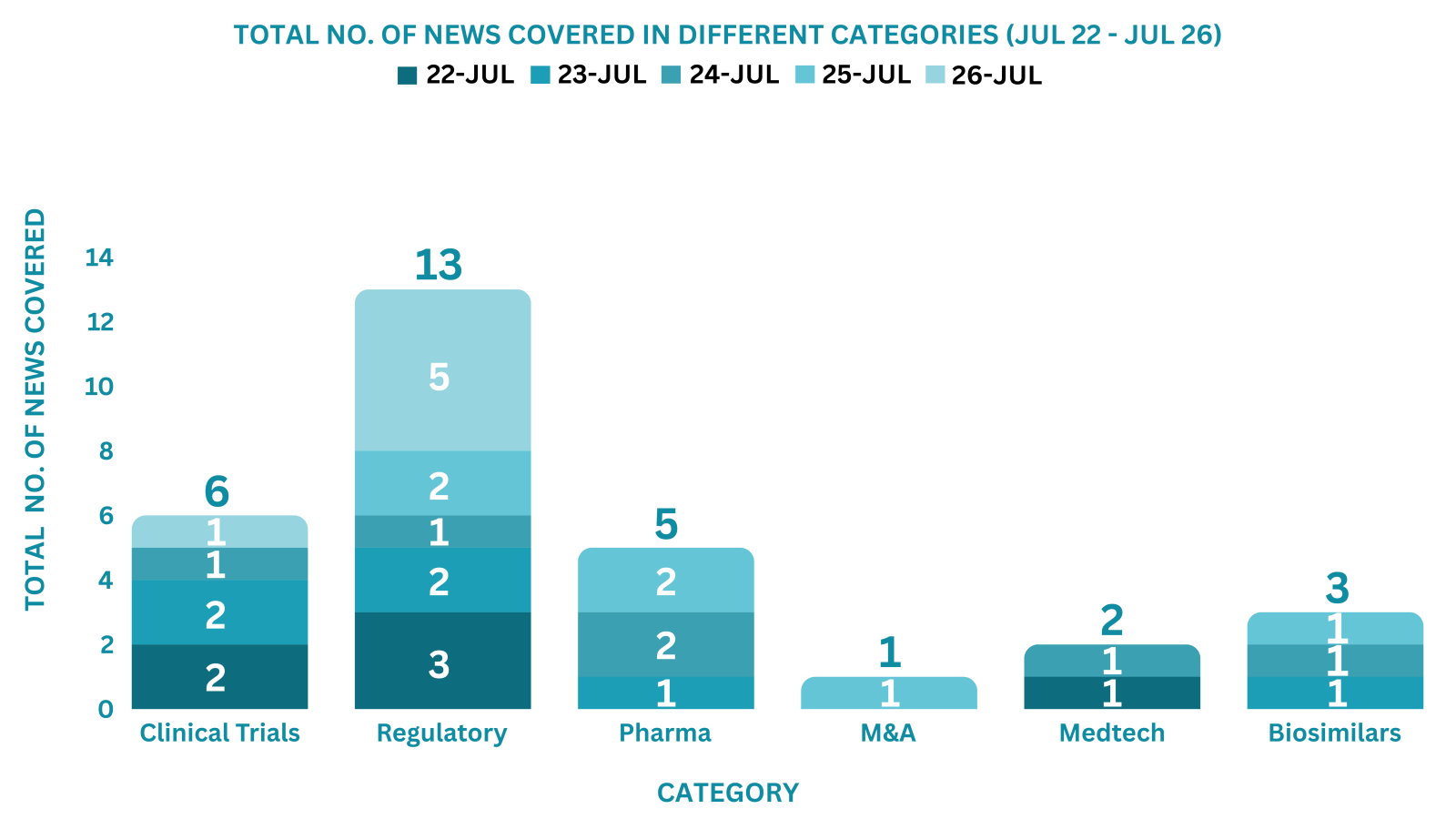
PharmaShots Weekly Snapshots (July 22 – July 26, 2024)
This week PharmaShots’ news was all about the updates on Clinical Trials, Pharma, Biotech, COVID-19, Regulatory & MedTech. Check out our full report below:

Innovent Reports Data from the P-III (DREAMS-1) Study of Mazdutide to Treat Type 2 Diabetes in Chinese Patients
Read More: Innovent
EDAP Reports Interim Data from P-III Trial of Robotic HIFU for Treating Deep Infiltrating Endometriosis
Read More: EDAP
Cytoki Pharma Reports Results from the P-I Study of CK-0045 to Improve Cardiometabolic Risk Factors
Read More: Cytoki Pharma
Merck Reports the P-IIb/III Study Data of Clesrovimab (MK-1654) to Prevent RSV Disease in Infants
Read More: Merck
Pfizer Reports Topline Data from P-III Trial of Giroctocogene Fitelparvovec for Treating Hemophilia A
Read More: Pfizer
Calliditas Reports Results from P-IIb (TRANSFORM) Study of Setanaxib for Treating Primary Biliary Cholangitis
Read More: Calliditas

Certa Therapeutics’ FT011 Gains the EMA’s Orphan Drug Designation to Treat Systemic Sclerosis
Read More: Certa Therapeutics
Zymeworks Reports the US FDA’s IND Clearance of ZW191 for Treating Solid Tumors
Read More: Zymeworks
Johnson & Johnson Reports sNDA Submission of Spravato to the US FDA for Treatment-Resistant Depression
Read More: Johnson & Johnson
The USPTO Issues Patent Covering Precision Biologics’ NEO-201 for its Methods of Targeting Treg Cells
Read More: Precision Biologics
AffyImmune’s AIC100 Gains the US FDA’s Regenerative Medicine Advanced Therapy (RMAT) Designation for Recurrent Anaplastic Thyroid Cancer
Read More: Affylmmune
Arcutis Reports the sNDA Submission of Zoryve Foam to the US FDA for Treating Scalp and Body Psoriasis
Read More: Arcutis
BioMarin Pharmaceutical Reports the US FDA’s Approval of Brineura (cerliponase alfa) to Treat Neuronal Ceroid Lipofuscinosis Type 2
Read More: BioMarin Pharmaceutical
ConSynance Therapeutics’ CSTI-500 Receives the US FDA’s Rare Pediatric Disease Designation to Treat Prader-Willi Syndrome
Read More: ConSynance Therapeutics
Pfizer Reports the EC’s Conditional Approval of Durveqtix (Fidanacogene Elaparvovec) for the Treatment of Hemophilia B
Read More: Pfizer
Sun Pharma’s Leqselvi (Deuruxolitinib) Receives the US FDA’s Approval for Treating Severe Alopecia Areata
Read More: Sun Pharma
AbbVie’s Skyrizi (Risankizumab) Receives the EC’s Approval to Treat Moderate to Severe Active Ulcerative Colitis
Read More: AbbVie
LEO Pharma’s Anzupgo (Delgocitinib) Gains the CHMP’s Positive Opinion to Treat Moderate to Severe Chronic Hand Eczema (CHE)
Read More: LEO Pharma
Merck Reports the CHMP’s Positive Opinion of Keytruda Plus Padcev to Treat Unresectable or Metastatic Urothelial Carcinoma
Read More: Merck

Aligos Therapeutics Partners with Xiamen Amoytop Biotech for the Development of ALG-000184
Read More: Aligos Therapeutics & Amoytop Biotech
Pinetree Therapeutics Collaborates with AstraZeneca to Develop its Preclinical Candidate
Read More: Pinetree Therapeutics & AstraZeneca
Triastek Partners with BioNTech to Develop 3D Printed Oral RNA Therapeutics
Read More: Triastek & BioNTech
Ipsen Collaborates with Day One Biopharmaceuticals for the commercialization of Tovorafenib to Treat Pediatric Low-Grade Glioma
Read More: Ipsen & Day One Biopharmaceuticals
Dren Bio Partners with Novartis to Discover and Develop Novel Targeted Myeloid Engagers for Cancer
Read More: Dren Bio & Novartis

Restore Medical’s ContraBand System Gains the US FDA’s Breakthrough Device Designation for Heart Failure with Reduced Ejection Fraction
Read More: Restore Medical
THINK Surgical Gains the US FDA’s Approval for TMINI Miniature Robotic System (TMINI 1.1)
Read More: THINK Surgical

Samsung Bioepis Reports the US FDA’s Approval of Epysqli (Biosimilar, Soliris)
Read More: Samsung Bioepis
Bio-Thera Reports the Regulatory Filing Acceptance for BAT2206 (Biosimilar, Stelara) Across the US and EU
Read More: Bio-Thera
Bio-Thera Solutions Reports the Initiation of Combined P-I/P-III Study of BAT3306 (Biosimilar, Keytruda)
Read More: Bio-Thera

Edwards Lifesciences to Acquire JenaValve Technology and Endotronix, Strengthening its Structural Heart Portfolio
Read More: Edwars Lifesciences, JenaValve Technology & Endotronix
Related Post: PharmaShots Weekly Snapshots (July 15 – July 19, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














